![]() Click to view abstract in PDF format.
Click to view abstract in PDF format.
Characterization and Application of Sorbed Gas by Microdesorption CF-IRMS
By
Michael J. Whiticar1
email: [email protected]
1Biogeochemical Facility School of Earth and Ocean Sciences (SEOS), University of Victoria P.O. Box 3020, Victoria, BC, V8W 2Y3, Canada
The old adage “The best way to find oil is to drill for it” remains as true today as before. However, the question of where to drill has been significantly improved by advances in geophysical and geological techniques. In some cases geochemistry comprises part of a comprehensive exploration program. Surface geochemical methodologies, including biological approaches, continue to be performed. Sorbed gas measurement, pioneered by L. Horwitz (1982) and W. Stahl et al. (1981), is amongst the most viable of the surface geochemical palette. We have made considerable advances in both the analytical and interpretative aspects of sorbed gas method.
Today, our vacuum-acid—mechanical microdesorption technique (Figure 1) replaces of the original ‘Blender’ with the advantages of speed, safety and significantly reduced material requirements. To further simplify and improve performance, we have replaced the original ‘offline’ partitioning and oxidation method with our Continuous Flow-Isotope Ratio Mass Spectrometry (CF-IRMS) system (Figure 2). This system consists of a gas chromatograph (GC) linked on-line to the IRMS via a capillary combustion oven, water removal component and splitter. An example of the CF-IRMS partitioning and detection “gas chromatogram” is shown in Figure 3a for the continuous monitoring of masses 44, 45, 46 (typically 12C16O16O, 13C16O16O, and 12C16O18O respectively). The 3-D integration is important to account for 17O and for the inherent isotope fractionation along the GC column. The figure shows that the important baseline separation between compounds is attained, including the NO2 (mass 44, 45 and 46 CO2 isobar) that results from the combustion of N2 (air) in the sample. The figure also shows the standard CO2 gas injections to reference the 13C/12C measurements to PDB. Figure 3b plots the resultant shifts in the mass 45/44 ratio trace for the individual compounds as they sequentially elute from the GC. The partial intracolumn isotope partitioning is observed in the classical isotope ratio swing.
Interpretatively, there have been substantial advances in our understanding of gas sorption phenomena, both in surface and subsurface environments, Our capability to characterize natural gases in reservoirs, cuttings and surface samples has especially been improved using the 13C/12C of higher hydrocarbon gases.
Gases in soils and sediments can physically occur in several phases. Some of these are operationally defined by their physical location and the analytical method to detect them (Figure 4), namely:
1. Free Gas – highly mobile, gaseous phase, typically in interstitial spaces or pores,
2. Dissolved Gas – gas in solution, e.g., dissolved in interstitial fluid, oil, condensates,
3. Inclusion Gas – largely immobile, trapped gases in fluid or crystal inclusions,
4. Sorbed Gas – Gases more strongly bound to mineral or organic solid surfaces. The mobility of sorbed gases is restricted.
Although the science of gas sorption phenomena is evolving rapidly, some of the questions related to the rates of gas exchange between the different phases remain uncertain. However, evidence indicates that the interaction between dissolved and sorbed gases is severely restricted. Therefore, for example, if a bacterial gas is located in the interstitial space of a sediment, i.e., a Free Gas, the exchange with a thermogenic on a sorbed gas site is insignificant. Although this is intuitively difficult to comprehend, there is strong isotope and molecular compositional evidence to support this.
Despite not having a complete understanding of the sorbed gas mechanism, there are several features found in previous work that makes sorbed gases attractive from a petroleum exploration perspective. For example, sorbed gases:
1. Generally carry the molecular and stable isotope signatures of the deeper seated hydrocarbons if present,
2. Do not readily exchange with free or dissolved gases in the interstitial spaces,
3. Are protected from microbial attack,
4. Migrate vertically perhaps by “handshake migration” (Figure 5). This is a process by which gases move vertically along surfaces without interacting with the adjacent pore space. This is a critical aspect of the use of sorbed gases as a tool to characterize deeper-seated hydrocarbon accumulations or active source rocks,
5. Structure or ordered water plays a key role in the isolation of sorbed gases from free gases, i.e., very restricted exchange or interaction of mineral or organic surfacebound gases with unbound interstitial gases (Figure 4). The structure water is in fact a relatively impermeable membrane of organized H2O molecules, actually very unlike conventional liquid “water”. Structure water forms a contiguous coating or network that extends the entire sediment column. This membrane reduces the sorbed-free gas exchange and promotes the lateral solid surface migration.
The emerging theories regarding the restriction of gas exchange interaction between dissolved and sorbed gases by the presence of “structure” or “ordered” H2O may hold the key to understanding the apparent contradictions of genetically different gases juxtaposed in the same sample.
This presentation will highlight these controversies and advances made in the application of sorbed gas Microdesorption CF-IRMS, and give examples of its application.
Figure Captions
 Figure
1. Microdesorption instrumentation.
Figure
1. Microdesorption instrumentation.
 Figure
2. Schematic of CF-IRMS system.
Figure
2. Schematic of CF-IRMS system.
![]() Figure
3. A). Mass 44, 45 and 46 gas chromatogram trace. B). Mass 45/44 isotope ratio
trace.
Figure
3. A). Mass 44, 45 and 46 gas chromatogram trace. B). Mass 45/44 isotope ratio
trace.
 Figure
4. Different gas types in sediments and soils.
Figure
4. Different gas types in sediments and soils.
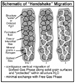 Figure
5. Handshake migration of sorbed gas.
Figure
5. Handshake migration of sorbed gas.
References Cited
Horvitz, L. 1982. Upward migraion of hydrocarbons from gas and oil deposits, 183rd Am. Chem. Soc. Nat. Mtg., Las Vegas, Nevada, March 28-April 2.
Stahl, W., E. Faber and D.L. Kirksey, 1981. Near-surface evidence of migration of natural gas from deep reservoirs and source rocks, AAPG Bull., v. 65, p. 1543-1550.
