AAPG/GSTT
HEDBERG CONFERENCE
“Mobile Shale
Basins – Genesis, Evolution and Hydrocarbon Systems “
Mud volcanoes –
a result of supercritical water formation at depth?
Martin Hovland1, Håkon Rueslåtten2,
Helge Løseth3, Christine Fichler3, Hans Konrad Johnsen3
1Statoil ASA,
2Numerical Rocks ASA,
3Statoil ASA,
Geological setting of mud volcanoes
Mud volcanoes occur in deep sedimentary basins located at relict or active plate boundaries. Some pertinent examples are:
-
Chandragup, located on the
Makran Accretionary
-
Abundant mud volcanoes in
-
Marine mud volcanoes, such as
the Campeche Knolls (shale/salt diapirs), in the
- Large mud volcanoes on the Mid Mediterranean Ridge, located on a subduction/accretionary system.
-
Mud volcanoes on and off
Mud volcanoes have been studied for more than
100 years. Hedberg (1974) concluded that they were consequences of
over-pressure caused by oil and gas generation at depth. A problem with this
hypothesis are the difficulties of explaining the formation of some of the
products that well up together with hydrocarbons in most mud volcanoes.
Products of mud volcanoes
In general, the terrestrial and ocean-bottom
mud volcanoes produce three main components: very fine-grained clayey material
(‘mud gel’), water of varying chlorinity, and hydrocarbons: both liquids and
gases (Brown, 1990; Hovland et al., 1997; Milkov, 2000; Planke et al., 2003).
Planke et al. (2003), found
that terrestrial mud volcanoes in Azerbaijan emitted brines with net additions
of B, Na, Al, Cr, Fe, Mn, Ni, Cu, Zn, As, Cd, Ba, U, Cl, and Br and a net
removal of Ca, Mg, K, and SO4, compared to seawater. This is despite
the Caspian Sea being an intra-continental drainage basin (draining the
Recently, a completely new type of
fluid-releasing piercement structure was found in the
A new formation hypothesis
Due to the lack of a convincing and unifying model for the formation of
the World’s numerous terrestrial and marine mud volcanoes, we are currently
examining the possible role of supercritical water formed at depth in
sedimentary basins. From observations of deep-sea hydrothermal vents, it is
known that ‘phase separation’ occurs (Bischoff and Rosenbauer, 1989). ‘Phase
separation’ is just another term for ‘supercritical water’, which forms at elevated
temperatures and pressures. For seawater, the supercritical point is around
Tc=405oC, and Pc=300 bars (equivalent to a seawater hydrostatic
pressure of 2,800 m water depth).
In deep sedimentary basins at depths beyond 10 km, it is conceivable
that porewaters can locally (and temporarily) achieve temperatures of 400oC
or more. At pressures above 300 bars, the water will become supercritical, with
all the ramifications involved. When the pressure is too great for water to
boil (> 221 bars for pure water, and > 300 bars for normal seawater), it
attains the supercritical state, which is neither vapor nor liquid, but
something in between. The
density of water changes rapidly with both temperature and pressure, and is
intermediate between that of liquid water (1 g/cm3) and low-pressure
water vapor (<0.001 g/cm3). At supercritical conditions, the
density is approximately 0.3 g/cm3 (Tester et al., 1993;
Bellissent-Funel, 2001). Similarly, the ionic dissociation constant falls from
10-14 at ambient conditoins to 10-23 in the supercritical
state. Furthermore, Raman spectra of
deuterated water in the supercritical region show only a small residual amount
of hydrogen bonding. As a result, the supercritical water acts essentially as a
non-polar, dense fluid, and its solvation properties resemble those of
low-polarity organic fluids (Tester et al., 1993). Supercritical water can
therefore be regarded as a non-polar fluid which is able to dissolve organic
liquids (oils), but unable to dissolve common sea salts (Armellini and Tester,
1991). There are also strong indications that supercritical water is corrosive
to silicate rocks (e.g., as seen from the precipitation of silica at venting
sites on the sea floor), a property which is of particular importance when
studying deep hydrothermal systems and alteration of rocks and sediments.
From scientific drilling performed by the Ocean Drilling Program (ODP),
it is also known that hydrothermal systems continue to be active even long
after they have been covered by sediments. This has been demonstrated in the
Besides the high pressure, a temperature of at least 405oC is
needed to form supercritical water. We believe this is achievable locally in
deep sedimentary basins (at depths beyond 10 km), e.g., by local high
heat-flows close to the basement due to the migration of hot fluids from active
hydrothermal systems. It is suggested that such supercritical point-sources at
depth would migrate upwards due to buoyancy.
We envisage that all the products: gases, liquids (oil and
water/brines), and a slurry of mud (with some clasts from the sidewall-rocks of
the conduit) start their long transit to surface in a slurry, driven by
supercritical water. However, upon cooling and decompression inside the
conduit, the water will enter into a two-phase state (with boiling). In this
situation we will also have a vapor-driven process, until it condenses upon
further adiabatic and conductive cooling (Fig. 1).
Other compounds, e.g., some hydrocarbons, will also condense to liquid
states, upon the upward migration. It is also expected that inorganic
components dissolved in the water and amorphous material in the slurry will
precipitate and/or crystallize during the ascent to surface. Such processes can
explain the migration of all the products associated with mud volcanoes, and
thus represent a unifying model for this type of natural phenomenon. Although
very few terrestrial mud volcanoes are known to have an elevated temperature
signature, some of the marine ones have. Thus, recent extreme heat-flow and
temperature values were measured on the Vodyanitskiy mud volcanoes in the
References
Armellini, F.J., Tester,
J.W., 1991. Experimental methods for studying salt nucleation and growth from
supercritical water. Journal of supercritical fluids 4, 254-264.
Bellissent-Funel, M.-C., 2001. Structure of supercritical water.
Journal Molecular Liquids 90, 313-322.
Bischoff, J.L. and Rosenbauer,
R.J., 1989. Salinity
variations in submarine hydrothermal systems by layered double-diffusive
convection. Journal of Geology 97, p. 613-623.
Brown, K.M., 1990.
The nature and hydrogeologic significance of mud diapirs and diatremes for
accretionary systems. Journal of Geophys. Res. 95, 8969–8982.
Hedberg, H. D., 1974. Relation of methane generation to
undercompacted shales, shale diapirs and mud volcanoes. AAPG Bull. 58, 661-673.
Hovland, M., Hill, A., Stokes, D., 1997. The
structure and geomorphology of the Dashgil mud volcano,
Hovland, M., MacDonald, I.R.,
Rueslåtten, H., Johnsen, H.K., Naehr,
T., Bohrmann, G., 2005. Was the Chapopote asphalt-volcano,
MacDonald, I.R., Bohrmann, G., Escobar, E.,
Abegg, F., Blanchon, P., Blinova, V., Brückmann, W., Drews, M., Eisenhauer, A.,
Han, X., Heeschen, K., Meier, F., Mortera, C., Naehr, T., Orcutt, B., Bernard,
B., Brooks, J., de Faragó, M., 2004. Asphalt volcanism and chemosynthetic life
in the Campeche Knolls,
Milkov, A.V.,
2000. Worldwide
distribution of submarine mud volcanoes and associated gas hydrates. Marine
Geol. 167, 29-42.
Planke, S., Svensen, H., Hovland, M., Banks,
D.A., Jamtveit, B., 2003. Mud and fluid migration in active mud volcanoes in
Poort, J.,Kutas, R., Vassilev, A., Klerkx, J.,
2005. Heat flow variability in the seep dominated nothern margin of the
Tester, J., Holgate, H.R., Armellini, F.J.,
Webley, P.A., Killilea, W.R., Hong, G.T., Berner.H.E., 1993. Supercritical
water oxidation technology. In: Emerging technologies in hazardous waste
management III, American Chemical Society, 35-76.
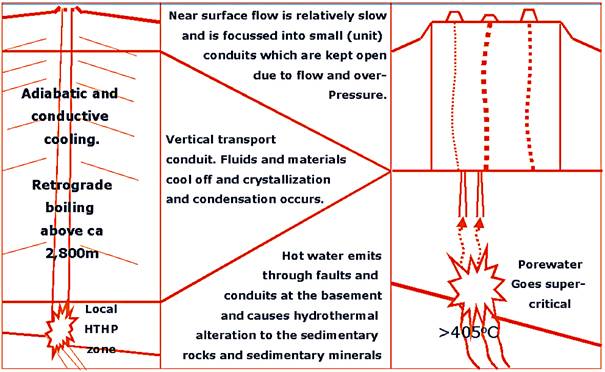
Fig. 1
A conceptual diagram, showing the most important
aspects of our new mud volcano formation model.
AAPG Search and Discovery Article #90057©2006 AAPG/GSTT Hedberg Conference, Port of Spain, Trinidad & Tobago
