|
uIntroduction
uFigure
Captions
uSubaerial
meteoric model
u Porosity Porosity in limestones
in limestones
u Porosity Porosity in dolomites
in dolomites
uCavernous
 porosity porosity
uMesogenetic
model
uMicroporosity
uConclusions
uReferences
uIntroduction
uFigure
Captions
uSubaerial
meteoric model
u Porosity Porosity in limestones
in limestones
u Porosity Porosity in dolomites
in dolomites
uCavernous
 porosity porosity
uMesogenetic
model
uMicroporosity
uConclusions
uReferences
uIntroduction
uFigure
Captions
uSubaerial
meteoric model
u Porosity Porosity in limestones
in limestones
u Porosity Porosity in dolomites
in dolomites
uCavernous
 porosity porosity
uMesogenetic
model
uMicroporosity
uConclusions
uReferences
uIntroduction
uFigure
Captions
uSubaerial
meteoric model
u Porosity Porosity in limestones
in limestones
u Porosity Porosity in dolomites
in dolomites
uCavernous
 porosity porosity
uMesogenetic
model
uMicroporosity
uConclusions
uReferences
uIntroduction
uFigure
Captions
uSubaerial
meteoric model
u Porosity Porosity in limestones
in limestones
u Porosity Porosity in dolomites
in dolomites
uCavernous
 porosity porosity
uMesogenetic
model
uMicroporosity
uConclusions
uReferences
uIntroduction
uFigure
Captions
uSubaerial
meteoric model
u Porosity Porosity in limestones
in limestones
u Porosity Porosity in dolomites
in dolomites
uCavernous
 porosity porosity
uMesogenetic
model
uMicroporosity
uConclusions
uReferences
uIntroduction
uFigure
Captions
uSubaerial
meteoric model
u Porosity Porosity in limestones
in limestones
u Porosity Porosity in dolomites
in dolomites
uCavernous
 porosity porosity
uMesogenetic
model
uMicroporosity
uConclusions
uReferences
uIntroduction
uFigure
Captions
uSubaerial
meteoric model
u Porosity Porosity in limestones
in limestones
u Porosity Porosity in dolomites
in dolomites
uCavernous
 porosity porosity
uMesogenetic
model
uMicroporosity
uConclusions
uReferences
uIntroduction
uFigure
Captions
uSubaerial
meteoric model
u Porosity Porosity in limestones
in limestones
u Porosity Porosity in dolomites
in dolomites
uCavernous
 porosity porosity
uMesogenetic
model
uMicroporosity
uConclusions
uReferences
uIntroduction
uFigure
Captions
uSubaerial
meteoric model
u Porosity Porosity in limestones
in limestones
u Porosity Porosity in dolomites
in dolomites
uCavernous
 porosity porosity
uMesogenetic
model
uMicroporosity
uConclusions
uReferences
uIntroduction
uFigure
Captions
uSubaerial
meteoric model
u Porosity Porosity in limestones
in limestones
u Porosity Porosity in dolomites
in dolomites
uCavernous
 porosity porosity
uMesogenetic
model
uMicroporosity
uConclusions
uReferences
uIntroduction
uFigure
Captions
uSubaerial
meteoric model
u Porosity Porosity in limestones
in limestones
u Porosity Porosity in dolomites
in dolomites
uCavernous
 porosity porosity
uMesogenetic
model
uMicroporosity
uConclusions
uReferences
uIntroduction
uFigure
Captions
uSubaerial
meteoric model
u Porosity Porosity in limestones
in limestones
u Porosity Porosity in dolomites
in dolomites
uCavernous
 porosity porosity
uMesogenetic
model
uMicroporosity
uConclusions
uReferences
|
Figures Captions
|
 |
Figure 1. Processes by which  porosity porosity is
reduced in is
reduced in  carbonate carbonate rocks. Syndepositional marine cementation
occurs only in the eogenetic zone, and mechanical compaction is
unlikely to affect telogenetically-exposed older rocks. Syndepositional marine cementation
occurs only in the eogenetic zone, and mechanical compaction is
unlikely to affect telogenetically-exposed older  carbonate carbonate rocks. rocks.
|
|
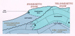 |
Figure 2. Meteoric subaerial exposure of
newly-deposited sediments in the eogenetic zone and of older
 carbonate carbonate rocks in the telogenetic zone. Note that the freshwater
lens in the eogenetic zone can extend some distance below
sea level ("B"), and that freshwater may
extend down -dip for a considerable distance in the telogenetic
zone ("A"). rocks in the telogenetic zone. Note that the freshwater
lens in the eogenetic zone can extend some distance below
sea level ("B"), and that freshwater may
extend down -dip for a considerable distance in the telogenetic
zone ("A"). |
|
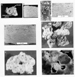 |
Figure 3. Typical secondary dissolution
pore types in  carbonate carbonate rocks that are readily identifiable in
cuttings and core samples. A - interparticle pores in a grainstone
(cuttings sample) and A' core showing interparticle rocks that are readily identifiable in
cuttings and core samples. A - interparticle pores in a grainstone
(cuttings sample) and A' core showing interparticle  porosity porosity in a in a
 carbonate carbonate grainstone. B - thin-section
photomicrograph of intraparticle grainstone. B - thin-section
photomicrograph of intraparticle  porosity porosity (arrows) within a
fusulinid and B' core showing intraparticle (arrows) within a
fusulinid and B' core showing intraparticle  porosity porosity within a
coral. C- Fenestral within a
coral. C- Fenestral  porosity porosity in a tidal-flat dolomite. Tilted
arrows point to planar (laminar)
fenestral pores, and horizontal arrows point to smaller "birdseye"
pores. D - Cuttings samples with oomoldic pores (arrows) in an
oolite grainstone. E - in a tidal-flat dolomite. Tilted
arrows point to planar (laminar)
fenestral pores, and horizontal arrows point to smaller "birdseye"
pores. D - Cuttings samples with oomoldic pores (arrows) in an
oolite grainstone. E -  Carbonate Carbonate grainstone with identifiable
skeletal particles (circled) and
larger vug (arrow) that formed from the initial dissolution of a
particle and then further dissolution of the matrix around it
(cuttings sample). F - Thin-section photomicrograph of
interparticle grainstone with identifiable
skeletal particles (circled) and
larger vug (arrow) that formed from the initial dissolution of a
particle and then further dissolution of the matrix around it
(cuttings sample). F - Thin-section photomicrograph of
interparticle  porosity porosity in a in a  carbonate carbonate sand wherein remnant cement (arrow) restricts pore throats and
reduces
sand wherein remnant cement (arrow) restricts pore throats and
reduces  permeability permeability . .
|
|
 |
Figure 4. Core slab with secondary vugs
(arrows) resulting from the partial dissolution of earlier
dolomite cement (white). |
|
 |
Figure 5. Thin-section photomicrograph of calcite cement
overgrowths on crinoid fragments that occlude interparticle
 porosity porosity . . |
|
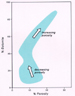 |
Figure 6. Relationship of  porosity porosity to
percent dolomite in to
percent dolomite in  carbonate carbonate rocks (after Murray, 1960). rocks (after Murray, 1960).
|
|
 |
Figure 7. Origin of secondary dissolution  porosity porosity in dolomites. in dolomites.
|
|
 |
Figure 8. Intercrystalline pores and pore
throats in dolomites. Relative size of pores and pore throats is
not necessarily correlative to dolomite crystal size because
 porosity porosity is a percentage of total rock volume. Pore throat
characteristics, however, do reflect the
degree (extent) of dolomitization in rocks. Dolomites with
polyhedral pores generally are referred to as "sucrosic." is a percentage of total rock volume. Pore throat
characteristics, however, do reflect the
degree (extent) of dolomitization in rocks. Dolomites with
polyhedral pores generally are referred to as "sucrosic." |
|
 |
Figure 9. Top - Typical lateral and
vertical compartmentalization of reservoir zones in cavernous
(karsted)  carbonate carbonate rocks. Bottom - Typical rocks. Bottom - Typical  porosity porosity types and
fills of cavernous reservoirs. Cave roof rocks become
progressively more brecciated downward,
with attending fracture, dissolution-enlarged fracture, and
commonly, vuggy types and
fills of cavernous reservoirs. Cave roof rocks become
progressively more brecciated downward,
with attending fracture, dissolution-enlarged fracture, and
commonly, vuggy  porosity porosity . Cave-fill deposits variously can be: (1)
cave roof-collapse breccia, which can have
inter-clast . Cave-fill deposits variously can be: (1)
cave roof-collapse breccia, which can have
inter-clast  porosity porosity as well as
intra-clast vuggy and fracture as well as
intra-clast vuggy and fracture  porosity porosity . Conversely, original
inter-clast . Conversely, original
inter-clast  porosity porosity can be filled with cements and/or shale, or
can be filled with porous sand; (2) impermeable shale infiltered
into the cavern from above; or (3)
porous or tight sand infiltered into the cavern from above. Cave
roof collapse and infiltering of sand and/or shale can occur soon
after karstification or later. can be filled with cements and/or shale, or
can be filled with porous sand; (2) impermeable shale infiltered
into the cavern from above; or (3)
porous or tight sand infiltered into the cavern from above. Cave
roof collapse and infiltering of sand and/or shale can occur soon
after karstification or later.
|
|
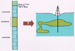 |
Figure 10. On the left is a partial
stratigraphic column of what I have sometimes encountered in the
Mississippian in Kansas a few feet of  carbonate carbonate below the top of
the Mississippian, underlain by sandstones (which
are not laterally correlative for any
distance), in turn underlain by more below the top of
the Mississippian, underlain by sandstones (which
are not laterally correlative for any
distance), in turn underlain by more  carbonate carbonate . On the right is a
possible interpretation of such a stratigraphy that the wells in
question encountered sand-filled caverns below the top of the
Mississippian. . On the right is a
possible interpretation of such a stratigraphy that the wells in
question encountered sand-filled caverns below the top of the
Mississippian. |
|
 |
Figure 11. Processes and products of hydrocarbon generation.
Relatively minor amounts of methane (CH4)
are generated during shallow burial by
bacterial processes. As organic matter
in source rocks matures and generates
oil or gas, copious amounts of hydrogen
sulfide (H2S) and  carbonate carbonate dioxide (CO2)
are generated, along with organic acids. dioxide (CO2)
are generated, along with organic acids.
|
|
 |
Figure 12. Carbon dioxide, hydrogen
sulfide, and organic acids evolved during hydrocarbon generation
mix with connate waters in the burial environment to produce
acids. These acidic fluids can migrate both vertically
and laterally for great distances, even
affecting  carbonate carbonate rocks that are not deeply buried (5). Acidic
fluids can migrate along faults (1), along bedding planes or
porous strata within beds (2), along formational boundaries (3),
or upward along fractures through
otherwise non-permeable beds ("cross-formational flow" (4). rocks that are not deeply buried (5). Acidic
fluids can migrate along faults (1), along bedding planes or
porous strata within beds (2), along formational boundaries (3),
or upward along fractures through
otherwise non-permeable beds ("cross-formational flow" (4).
|
|
 |
Figure 13. Mesogenetic  porosity porosity . A -
Fractures, which cross and therefore post-date stylolites, with . A -
Fractures, which cross and therefore post-date stylolites, with
 porosity porosity from the dissolution of post-fracture cement. Adjoining
this area is selective dissolution of oolites. B - Oomoldic from the dissolution of post-fracture cement. Adjoining
this area is selective dissolution of oolites. B - Oomoldic
 porosity porosity adjoining and post-dating the
formation of a stylolite. adjoining and post-dating the
formation of a stylolite. |
|
 |
Figure 14. Mesogenetic  porosity porosity . A -
Thin-section photomicrograph of curved saddle dolomite (with
typical undulose extinction) filling a pore. B - Core slab showing
coarse crystalline saddle dolomite that has been partially
dissolved to exhume (vuggy) . A -
Thin-section photomicrograph of curved saddle dolomite (with
typical undulose extinction) filling a pore. B - Core slab showing
coarse crystalline saddle dolomite that has been partially
dissolved to exhume (vuggy)  porosity porosity
|
Return to top.
Secondary  Porosity Porosity Beneath Unconformities:
The Subaerial Meteoric Model Beneath Unconformities:
The Subaerial Meteoric Model
The
generation of secondary  porosity porosity in in  carbonate carbonate sediments or rocks in this
model is a direct
consequence of dissolution by
freshwater (ultimately rain-derived), which dissolves carbonates because
the water is undersaturated with respect to calcium sediments or rocks in this
model is a direct
consequence of dissolution by
freshwater (ultimately rain-derived), which dissolves carbonates because
the water is undersaturated with respect to calcium  carbonate carbonate . The
extent of dissolution and secondary . The
extent of dissolution and secondary  porosity porosity formation are controlled by
factors such as the acidity of freshwater (e.g., rain water percolating
down through a soil zone will be more acidic than in areas where soils
are not present), the amount of formation are controlled by
factors such as the acidity of freshwater (e.g., rain water percolating
down through a soil zone will be more acidic than in areas where soils
are not present), the amount of  porosity porosity or fractures within the
affected carbonates, the residence time of the freshwater in the
diagenetic system, the mineralogy of the sediments or rocks, and so
forth (Longman, 1980; Moore, 1989). Secondary or fractures within the
affected carbonates, the residence time of the freshwater in the
diagenetic system, the mineralogy of the sediments or rocks, and so
forth (Longman, 1980; Moore, 1989). Secondary  porosity porosity generation via
dissolution can occur relatively soon after deposition, in
unconsolidated sediments, in what Choquette and Pray (1970) refer to as
the eogenetic zone; or it can occur much later, in rocks, in the
telogenetic zone as a consequence of uplift of older, formerly
buried carbonates (Figure 2). In newly-deposited generation via
dissolution can occur relatively soon after deposition, in
unconsolidated sediments, in what Choquette and Pray (1970) refer to as
the eogenetic zone; or it can occur much later, in rocks, in the
telogenetic zone as a consequence of uplift of older, formerly
buried carbonates (Figure 2). In newly-deposited  carbonate carbonate sediments
that subsequently are subaerially exposed, it is the difference in
original mineralogies of particles in the sediments that drives the
relatively rapid, selective dissolution of particles. Fragments of
corals, pelecypods and gastropods, and oolites, for example, are
originally composed mostly of the mineral aragonite (CaCO3,
orthorhombic), which is very soluble. It is for this reason that
formerly aragonitic particles in limestones usually are represented by
pores (in this case, fabric-selective pores) or cement-filled pores. In
contrast, particles such as forams, crinoid fragments, and bryozoans are
originally composed of the mineral high-magnesium calcite (CaCO3,
hexagonal-rhombohedral), which is calcite with up to 23 mole% MgCO3
in the
crystal lattice. With exposure to freshwater such particles tend merely
to lose MgCO3
and not to dissolve like
aragonitic particles. Other particles, such as brachiopod shells and
some pelecypods, build their skeletons out of low-Mg calcite (also CaCO3,
hexagonal-rhombohedral), which is calcite with less than 4 mole% MgCO3.
Particles of original high-magnesium calcite and low-magnesium calcite
mineralogy tend not to dissolve unless the freshwater is quite
undersaturated with respect to calcium sediments
that subsequently are subaerially exposed, it is the difference in
original mineralogies of particles in the sediments that drives the
relatively rapid, selective dissolution of particles. Fragments of
corals, pelecypods and gastropods, and oolites, for example, are
originally composed mostly of the mineral aragonite (CaCO3,
orthorhombic), which is very soluble. It is for this reason that
formerly aragonitic particles in limestones usually are represented by
pores (in this case, fabric-selective pores) or cement-filled pores. In
contrast, particles such as forams, crinoid fragments, and bryozoans are
originally composed of the mineral high-magnesium calcite (CaCO3,
hexagonal-rhombohedral), which is calcite with up to 23 mole% MgCO3
in the
crystal lattice. With exposure to freshwater such particles tend merely
to lose MgCO3
and not to dissolve like
aragonitic particles. Other particles, such as brachiopod shells and
some pelecypods, build their skeletons out of low-Mg calcite (also CaCO3,
hexagonal-rhombohedral), which is calcite with less than 4 mole% MgCO3.
Particles of original high-magnesium calcite and low-magnesium calcite
mineralogy tend not to dissolve unless the freshwater is quite
undersaturated with respect to calcium  carbonate carbonate , and it is for this
reason that some particles (crinoids, bryozoans, brachiopods) often are
well-preserved in ancient rocks. The eogenetic exposure to freshwater of
newly-deposited , and it is for this
reason that some particles (crinoids, bryozoans, brachiopods) often are
well-preserved in ancient rocks. The eogenetic exposure to freshwater of
newly-deposited  carbonate carbonate sediments, which are generally highly porous
and polyminerallic (i.e., as discussed above, composed of
mixtures of aragonite, high-magnesium and low-magnesium calcite),
results in the formation of cemented limestones, of varying sediments, which are generally highly porous
and polyminerallic (i.e., as discussed above, composed of
mixtures of aragonite, high-magnesium and low-magnesium calcite),
results in the formation of cemented limestones, of varying  porosity porosity , of
stable low-magnesium calcite composition (notwithstanding dolomitization).
In contrast, late eogenetic or telogenetic freshwater exposure of older
limestones that have already been mineralogically stabilized and
cemented is not driven by such differences in the relative solubility of
aragonite, high-magnesium and low-magnesium calcite because the rocks
already are mineralogically stabilized to low-magnesium calcite, and
further dissolution can occur only if the fluids are quite
undersaturated with respect to calcite (the least soluble of the
aforementioned , of
stable low-magnesium calcite composition (notwithstanding dolomitization).
In contrast, late eogenetic or telogenetic freshwater exposure of older
limestones that have already been mineralogically stabilized and
cemented is not driven by such differences in the relative solubility of
aragonite, high-magnesium and low-magnesium calcite because the rocks
already are mineralogically stabilized to low-magnesium calcite, and
further dissolution can occur only if the fluids are quite
undersaturated with respect to calcite (the least soluble of the
aforementioned  carbonate carbonate minerals). Typically, such dissolution forms
vugs and caverns, which can also form in polyminerallic minerals). Typically, such dissolution forms
vugs and caverns, which can also form in polyminerallic  carbonate carbonate sediments. Dissolution of already stabilized limestones can also result
in the formation of particle-selective pores when certain particles in
the rocks are slightly more soluble than other particles because of
differences in particle size or their micro-architectural arrangement of
component calcite crystals. For example, crinoid fragments in older
rocks are composed of single, relatively large crystals of low-magnesium
calcite, which have relatively low solubility. It is for this reason
that crinoid-rich Mississippian limestones, for example, typically have
low porosities. In contrast, foram shells are composed of myriads of
small calcite crystals, which have relatively higher solubility, and
they usually are more readily dissolved than crinoid fragments.
sediments. Dissolution of already stabilized limestones can also result
in the formation of particle-selective pores when certain particles in
the rocks are slightly more soluble than other particles because of
differences in particle size or their micro-architectural arrangement of
component calcite crystals. For example, crinoid fragments in older
rocks are composed of single, relatively large crystals of low-magnesium
calcite, which have relatively low solubility. It is for this reason
that crinoid-rich Mississippian limestones, for example, typically have
low porosities. In contrast, foram shells are composed of myriads of
small calcite crystals, which have relatively higher solubility, and
they usually are more readily dissolved than crinoid fragments.
In either
case, it is important to note that carbonates can be affected by
meteoric dissolution not only directly beneath unconformities on land,
but also for some distance down-dip into the subsurface ("A" in
Figure 2) and some distance in a seaward direction below sea level, depending
on the extent of freshwater lenses ("B" in
Figure 2).  Porosity Porosity generation by dissolution eventually ceases, generally in a down-dip
direction within phreatic zones when that water becomes saturated with
respect to dissolved calcium
generation by dissolution eventually ceases, generally in a down-dip
direction within phreatic zones when that water becomes saturated with
respect to dissolved calcium  carbonate carbonate . At that point, . At that point,  porosity porosity can be
maintained, or if the water becomes even more saturated with respect to
dissolved calcium can be
maintained, or if the water becomes even more saturated with respect to
dissolved calcium  carbonate carbonate , it can begin to be occluded by , it can begin to be occluded by  carbonate carbonate cement (and other cements as well, such as gypsum/anhydrite or silica).
cement (and other cements as well, such as gypsum/anhydrite or silica).
Meteoric  Porosity Porosity in
Limestones in
Limestones
In
limestones, common secondary pore types formed as a result of
post-depositional dissolution variously include exhumed interparticle,
intraparticle, fenestral, shelter, and growth-framework pores, all of
which are considered to be fabric-selective pores; and also not
fabric-selective vugs (Figure 3E) and dissolution-enlarged fractures.
The size of vugs (Figure 4) varies from small (but larger than component
particles in the rocks) to caverns or cavernous  porosity porosity . Vugs may
originate either by wholescale dissolution of parts of the rock or by
dissolutional enlargement of fabric-selective pores (Figure 3E). In many
cases there is coincidence between the types of fabric-selective pores
present in the rocks and the depositional environment of the rocks,
which serves as an important guide in evaluating . Vugs may
originate either by wholescale dissolution of parts of the rock or by
dissolutional enlargement of fabric-selective pores (Figure 3E). In many
cases there is coincidence between the types of fabric-selective pores
present in the rocks and the depositional environment of the rocks,
which serves as an important guide in evaluating  permeability permeability and
potential recoverable reserves from the reservoir, and in deciding on
what stimulation procedures to use but only if one knows the
depositional environment of the rocks from study of subsurface samples.
For example, and
potential recoverable reserves from the reservoir, and in deciding on
what stimulation procedures to use but only if one knows the
depositional environment of the rocks from study of subsurface samples.
For example,  carbonate carbonate sands (lime grainstones), deposited in
high-energy environments such as oolite shoals or skeletal sand shoals,
commonly have high interparticle sands (lime grainstones), deposited in
high-energy environments such as oolite shoals or skeletal sand shoals,
commonly have high interparticle  porosity porosity (Figure 3A) and attending
relatively high permeabilities. On the other hand, however, high (Figure 3A) and attending
relatively high permeabilities. On the other hand, however, high
 porosity porosity but low but low  permeability permeability may characterize a may characterize a  carbonate carbonate sand
(limestone) reservoir wherein only the particles have been dissolved
(for example, in cases where the reservoir contains only molds of
oolites referred to as oomoldic sand
(limestone) reservoir wherein only the particles have been dissolved
(for example, in cases where the reservoir contains only molds of
oolites referred to as oomoldic  porosity porosity or by the older term oocastic or by the older term oocastic
 porosity porosity : Figure 3D). In such cases there may be ample hydrocarbon
storage volume in the pores in the rocks, but in the absence of
fractures, there is little interconnected : Figure 3D). In such cases there may be ample hydrocarbon
storage volume in the pores in the rocks, but in the absence of
fractures, there is little interconnected  porosity porosity . Notwithstanding . Notwithstanding
 porosity porosity associated with dolomitization (discussed later), limestones
deposited in tidal-flat environments commonly contain a specific type of
vuggy associated with dolomitization (discussed later), limestones
deposited in tidal-flat environments commonly contain a specific type of
vuggy  porosity porosity referred to as fenestral pores (for example, birdseye
pores, which is one type of pinpoint referred to as fenestral pores (for example, birdseye
pores, which is one type of pinpoint  porosity porosity : Figure 3C), and unless
fractured, such rocks may have decent : Figure 3C), and unless
fractured, such rocks may have decent  porosity porosity but limited but limited  permeability permeability .
Skeletal sands shoals wherein the particles mainly are foraminifera,
which are common in Midcontinent Pennsylvanian limestone reservoirs (Wilhite
and Mazzullo, 2000), may be highly porous and permeable because of the
presence of interparticle pores, and within the forams, of intraparticle
pores as well (Figure 3B). On the other hand, if intraparticle pores are
the only pore types present, then .
Skeletal sands shoals wherein the particles mainly are foraminifera,
which are common in Midcontinent Pennsylvanian limestone reservoirs (Wilhite
and Mazzullo, 2000), may be highly porous and permeable because of the
presence of interparticle pores, and within the forams, of intraparticle
pores as well (Figure 3B). On the other hand, if intraparticle pores are
the only pore types present, then  porosity porosity (and hydrocarbon storage
volume) might be high, but (and hydrocarbon storage
volume) might be high, but  permeability permeability would be low (notwithstanding
fracturing). As a corollary, variations in would be low (notwithstanding
fracturing). As a corollary, variations in  porosity porosity and and  permeability permeability from well-to-well within a given zone may be a consequence of different
depositional environments in that zone and/or from differing extents of
from well-to-well within a given zone may be a consequence of different
depositional environments in that zone and/or from differing extents of
 porosity porosity generation versus occlusion between wells. Only study of
cores/cuttings and thin-sections can resolve the possible reasons for
such variations between wells. generation versus occlusion between wells. Only study of
cores/cuttings and thin-sections can resolve the possible reasons for
such variations between wells.
Over-riding such generalizations about the relationships among pore
types,  permeability permeability , and depositional environments of the limestones is
the importance of pore throats in the rocks (Wardlaw, 1976). In
limestones, particularly in grainstones, for example, the nature of pore
throats and their effect on , and depositional environments of the limestones is
the importance of pore throats in the rocks (Wardlaw, 1976). In
limestones, particularly in grainstones, for example, the nature of pore
throats and their effect on  permeability permeability is controlled by the size of
the particles in the rocks, and more importantly, by the distribution of
any remaining earlier-precipitated cement in the pores that was not
dissolved (Figure 3F). Calcite cement overgrowths on crinoid fragments
can significantly restrict pore throats as well (Figure 5), which is why
many crinoid-rich Mississippian limestones are of low- is controlled by the size of
the particles in the rocks, and more importantly, by the distribution of
any remaining earlier-precipitated cement in the pores that was not
dissolved (Figure 3F). Calcite cement overgrowths on crinoid fragments
can significantly restrict pore throats as well (Figure 5), which is why
many crinoid-rich Mississippian limestones are of low- permeability permeability nature. The best way to determine the extent of pore-throat restriction
in the rocks under consideration is by examining the rocks
petrographically in thin-section. Clay-mineral cements are extremely
rare in
nature. The best way to determine the extent of pore-throat restriction
in the rocks under consideration is by examining the rocks
petrographically in thin-section. Clay-mineral cements are extremely
rare in  carbonate carbonate reservoir rocks, and therefore, need not be considered
here. reservoir rocks, and therefore, need not be considered
here.
Meteoric  Porosity Porosity in
Dolomites in
Dolomites
In
contrast to earlier postulates on the subject (e.g., Murray, 1960; Weyl,
1960), the process of dolomitization of a pre-existing limestone does
not automatically create secondary  porosity porosity . Whereas it is true that . Whereas it is true that
 porosity porosity tends to increase as amount of dolomite increases (Figure 6),
it generally does so for the following reasons. In partly dolomitized
limestones exposed to telogenetic meteoric fluids, for example, any
remaining calcite (which may represent particles and/or tends to increase as amount of dolomite increases (Figure 6),
it generally does so for the following reasons. In partly dolomitized
limestones exposed to telogenetic meteoric fluids, for example, any
remaining calcite (which may represent particles and/or  carbonate carbonate mud
matrix) inherently is more susceptible to dissolution by freshwater
because it is more soluble than dolomite. Hence, subaerial exposure of a
partly dolomitized limestone can result in the generation of the same
types of pores as described above by dissolution of remaining calcite,
depending on the original texture of the rock (mudstone, wackestone,
packstone, or grainstone), its depositional environment, and degree of
replacement by dolomite (Figure 7). Likewise, remaining evaporites in
the rocks can also be dissolved. In more pervasively dolomitized rock
exposed to telogenetic meteoric fluids, remaining calcite (or evaporite
minerals) between dolomite crystals can be dissolved during subaerial
exposure to produce intercrystalline pores between dolomite crystals. In
completely dolomitized rocks, vugs (and sometimes dissolution-
enlarged
fractures) are common pore types present if the meteoric fluids were
highly acidic or acted on the rocks over long periods of time. Selective
dissolution of small dolomite crystals (because solubility increases as
crystal size decreases), or of more soluble dolomite phases in the
rocks, can result in the development of vugs and intercrystalline pores.
All such processes and resulting pore types can be represented in a
given reservoir. As in limestones, the nature of pore throats in
dolomites affects mud
matrix) inherently is more susceptible to dissolution by freshwater
because it is more soluble than dolomite. Hence, subaerial exposure of a
partly dolomitized limestone can result in the generation of the same
types of pores as described above by dissolution of remaining calcite,
depending on the original texture of the rock (mudstone, wackestone,
packstone, or grainstone), its depositional environment, and degree of
replacement by dolomite (Figure 7). Likewise, remaining evaporites in
the rocks can also be dissolved. In more pervasively dolomitized rock
exposed to telogenetic meteoric fluids, remaining calcite (or evaporite
minerals) between dolomite crystals can be dissolved during subaerial
exposure to produce intercrystalline pores between dolomite crystals. In
completely dolomitized rocks, vugs (and sometimes dissolution-
enlarged
fractures) are common pore types present if the meteoric fluids were
highly acidic or acted on the rocks over long periods of time. Selective
dissolution of small dolomite crystals (because solubility increases as
crystal size decreases), or of more soluble dolomite phases in the
rocks, can result in the development of vugs and intercrystalline pores.
All such processes and resulting pore types can be represented in a
given reservoir. As in limestones, the nature of pore throats in
dolomites affects  permeability permeability , and as a general rule, intercrystalline
pore throat sizes decrease with decreasing crystal size and extent of
dolomitization (Figure 8). , and as a general rule, intercrystalline
pore throat sizes decrease with decreasing crystal size and extent of
dolomitization (Figure 8).
Cavernous  Porosity Porosity in in
 Carbonate Carbonate Rocks Rocks
Cavernous
and associated vuggy  porosity porosity are major attributes of hydrocarbon
production from reservoirs such as the Arbuckle Group in Kansas
(Walters, 1946; Newell et al., 1987) and Oklahoma (Gatewood, 1970), and
from its stratigraphic correlative, the Ellenburger Group, in west Texas
and New Mexico (Holtz and Kerans, 1992). Additional examples of
hydrocarbon reservoirs in paleo-caverns are given in Mazzullo and
Chilingarian (1996). Only rarely are completely fluid-filled caverns
encountered in the subsurface. Rather, paleo-caverns usually are filled
by porous (or, unfortunately, sometimes tight) cave-roof collapse
breccia and associated sediments and/or by overlying, younger rocks
(Figure 9). Rather than being single zones, paleo-caverns typically are
labyrinthine systems characterized by extreme lateral and vertical
reservoir compartmentalization (Figure 9). Cavernous are major attributes of hydrocarbon
production from reservoirs such as the Arbuckle Group in Kansas
(Walters, 1946; Newell et al., 1987) and Oklahoma (Gatewood, 1970), and
from its stratigraphic correlative, the Ellenburger Group, in west Texas
and New Mexico (Holtz and Kerans, 1992). Additional examples of
hydrocarbon reservoirs in paleo-caverns are given in Mazzullo and
Chilingarian (1996). Only rarely are completely fluid-filled caverns
encountered in the subsurface. Rather, paleo-caverns usually are filled
by porous (or, unfortunately, sometimes tight) cave-roof collapse
breccia and associated sediments and/or by overlying, younger rocks
(Figure 9). Rather than being single zones, paleo-caverns typically are
labyrinthine systems characterized by extreme lateral and vertical
reservoir compartmentalization (Figure 9). Cavernous  porosity porosity undoubtedly also locally contributes to hydrocarbon production from some
Mississippian reservoirs in Kansas. I have encountered a number of
instances in Kansas, for example, where wellsite geologists’ reports
picked the top of the Mississippian at a certain depth, and then the
limestone or dolomite directly below seemingly was underlain by a
section of sand (which I presume to be Pennsylvanian-age siliciclastic
sand) that is, in turn, underlain by more
undoubtedly also locally contributes to hydrocarbon production from some
Mississippian reservoirs in Kansas. I have encountered a number of
instances in Kansas, for example, where wellsite geologists’ reports
picked the top of the Mississippian at a certain depth, and then the
limestone or dolomite directly below seemingly was underlain by a
section of sand (which I presume to be Pennsylvanian-age siliciclastic
sand) that is, in turn, underlain by more  carbonate carbonate rock. Such
occurrences may indicate that the wells penetrated sand-filled paleo-caverns
(Figure 10). rock. Such
occurrences may indicate that the wells penetrated sand-filled paleo-caverns
(Figure 10).
Since the
late 1970s-early 1980s, geologists began to suspect that not all
secondary dissolution  porosity porosity in in  carbonate carbonate rocks forms or formed solely
beneath unconformities by freshwater dissolution in either the eogenetic
or telogenetic environments (e.g., Bathurst, 1980; Scholle and Halley,
1985; Choquette and James, 1987; Moore, 1989). Rather, there was growing
realization of the significance of, and processes controlling, secondary
dissolution rocks forms or formed solely
beneath unconformities by freshwater dissolution in either the eogenetic
or telogenetic environments (e.g., Bathurst, 1980; Scholle and Halley,
1985; Choquette and James, 1987; Moore, 1989). Rather, there was growing
realization of the significance of, and processes controlling, secondary
dissolution  porosity porosity formation (and formation (and  porosity porosity occlusion) in the
deep-burial environment B which is what Choquette and Pray (1970)
referred to as the mesogenetic environment. Two important points
in this regard are the facts that: (1) not all porous carbonates are
associated with unconformities; and (2) specifically, there are a number
of examples of porous and permeable occlusion) in the
deep-burial environment B which is what Choquette and Pray (1970)
referred to as the mesogenetic environment. Two important points
in this regard are the facts that: (1) not all porous carbonates are
associated with unconformities; and (2) specifically, there are a number
of examples of porous and permeable  carbonate carbonate rocks deposited in
deep-water settings and which later were deeply buried and never
subaerially exposed. Hence, meteoric exposure at any time after
deposition has been ruled out for such rocks (e.g., Mazzullo and Harris,
1991; Mazzullo, 1994). Therefore, post-depositional diagenesis and the
formation of secondary dissolution rocks deposited in
deep-water settings and which later were deeply buried and never
subaerially exposed. Hence, meteoric exposure at any time after
deposition has been ruled out for such rocks (e.g., Mazzullo and Harris,
1991; Mazzullo, 1994). Therefore, post-depositional diagenesis and the
formation of secondary dissolution  porosity porosity in such rocks must have
occurred in an environment other than the meteoric eogenetic or
telogenetic environment. Furthermore, if we are exploring for
hydrocarbon reservoirs in the subsurface, then those reservoirs must
have resided in the subsurface, variously shallowly or deeply buried,
for long periods of time. Insofar as in such rocks must have
occurred in an environment other than the meteoric eogenetic or
telogenetic environment. Furthermore, if we are exploring for
hydrocarbon reservoirs in the subsurface, then those reservoirs must
have resided in the subsurface, variously shallowly or deeply buried,
for long periods of time. Insofar as  carbonate carbonate diagenesis never ceases,
any diagenesis that occurs in the mesogenetic environment overprints
earlier diagenesis, including that which may have occurred, for example,
in subunconformity, meteoric eogenetic or telogenetic environments.
Geologists have since come to realize that deep-burial diagenesis has
significantly contributed to secondary dissolution diagenesis never ceases,
any diagenesis that occurs in the mesogenetic environment overprints
earlier diagenesis, including that which may have occurred, for example,
in subunconformity, meteoric eogenetic or telogenetic environments.
Geologists have since come to realize that deep-burial diagenesis has
significantly contributed to secondary dissolution  porosity porosity and and
 permeability permeability evolution in many evolution in many  carbonate carbonate hydrocarbon reservoirs (e.g.,
Mazzullo and Harris, 1992). hydrocarbon reservoirs (e.g.,
Mazzullo and Harris, 1992).
As
discussed above, in order for dissolution of any  carbonate carbonate rocks to
proceed, they must be exposed to fluids that are undersaturated with
respect to calcium rocks to
proceed, they must be exposed to fluids that are undersaturated with
respect to calcium  carbonate carbonate . That is easy enough to do in the
subunconformity meteoric environment because rain water, the ultimate
source of near-surface freshwater, is undersaturated. In the mesogenetic
environment, however, most connate fluids are brines that typically are
saturated or even supersaturated with respect to calcium . That is easy enough to do in the
subunconformity meteoric environment because rain water, the ultimate
source of near-surface freshwater, is undersaturated. In the mesogenetic
environment, however, most connate fluids are brines that typically are
saturated or even supersaturated with respect to calcium  carbonate carbonate ,
which means they are not capable of dissolving ,
which means they are not capable of dissolving  carbonate carbonate rocks and
creating secondary rocks and
creating secondary  porosity porosity . Rather, such fluids tend to precipitate
carbonates in the form of calcite or dolomite cement, and in some cases,
they may be capable of dolomitization. How, then, can . Rather, such fluids tend to precipitate
carbonates in the form of calcite or dolomite cement, and in some cases,
they may be capable of dolomitization. How, then, can  carbonate carbonate dissolution and
dissolution and  porosity porosity formation proceed in the deep-burial
environment? In other words, how are fluids undersaturated with respect
to calcium formation proceed in the deep-burial
environment? In other words, how are fluids undersaturated with respect
to calcium  carbonate carbonate generated in the mesogenetic environment? generated in the mesogenetic environment?
Studies of
 porosity porosity evolution in sandstones, combined with studies of organic
matter maturation and hydrocarbon generation in source rocks (e.g.,
Foscolos, 1984; Surdam et al., 1984; Kharaka et al., 1986; Lundegard and
Land, 1986; Meshri, 1986; Sassen and Moore,
1988),
have provided answers to these questions which have been applied to evolution in sandstones, combined with studies of organic
matter maturation and hydrocarbon generation in source rocks (e.g.,
Foscolos, 1984; Surdam et al., 1984; Kharaka et al., 1986; Lundegard and
Land, 1986; Meshri, 1986; Sassen and Moore,
1988),
have provided answers to these questions which have been applied to
 carbonate carbonate reservoir rocks, both limestone and dolomite, around the world
(e.g., Druckman and Moore, 1985; Heydari and Moore, 1989; Mazzullo and
Harris, 1992). It is known that carbon dioxide, hydrogen sulfide, and
great quantities of organic acids are generated during the maturation of
organic matter to hydrocarbons in buried source rocks (Figure 11). As
these gases and organic acids are expelled from the source rock, the
evolved CO2
combines
with subsurface water to produce carbonic acid and the H2S
similarly combines with water to produce sulfuric acid. Together, these
acids and associated organic acids can migrate great distances laterally
as well as vertically (Figure 12; e.g., Hanor, 1987) to dissolve buried
carbonates just ahead of migrating hydrocarbons. Likewise, once the
acids are spent, subsurface fluids can then precipitate reservoir rocks, both limestone and dolomite, around the world
(e.g., Druckman and Moore, 1985; Heydari and Moore, 1989; Mazzullo and
Harris, 1992). It is known that carbon dioxide, hydrogen sulfide, and
great quantities of organic acids are generated during the maturation of
organic matter to hydrocarbons in buried source rocks (Figure 11). As
these gases and organic acids are expelled from the source rock, the
evolved CO2
combines
with subsurface water to produce carbonic acid and the H2S
similarly combines with water to produce sulfuric acid. Together, these
acids and associated organic acids can migrate great distances laterally
as well as vertically (Figure 12; e.g., Hanor, 1987) to dissolve buried
carbonates just ahead of migrating hydrocarbons. Likewise, once the
acids are spent, subsurface fluids can then precipitate  carbonate carbonate cements, which is why many examples of such cements contain hydrocarbon
inclusions (e.g., Burruss et al., 1985). In given rocks, secondary
dissolution
cements, which is why many examples of such cements contain hydrocarbon
inclusions (e.g., Burruss et al., 1985). In given rocks, secondary
dissolution  porosity porosity formation can alternate with cementation many times
to result in complex diagenetic histories of reservoirs (e.g., Moore and
Druckman, 1981; Mazzullo and Harris, 1989; Moore, 1989). Because such
evolved subsurface fluids can migrate great distances both laterally and
vertically, they can affect carbonates that were or are not deeply
buried. I have come across several wells in Ness County, Kansas, for
example, where there is a great concentration of pyrite and minor
sphalerite at the top of the Mississippian, which is at only about 4100
feet below the surface, clearly not in the deep-burial environment (at
least, not today). These minerals may have been emplaced along the
pre-Pennsylvanian unconformity by fluids that evolved within and
subsequently migrated out of the Anadarko Basin. formation can alternate with cementation many times
to result in complex diagenetic histories of reservoirs (e.g., Moore and
Druckman, 1981; Mazzullo and Harris, 1989; Moore, 1989). Because such
evolved subsurface fluids can migrate great distances both laterally and
vertically, they can affect carbonates that were or are not deeply
buried. I have come across several wells in Ness County, Kansas, for
example, where there is a great concentration of pyrite and minor
sphalerite at the top of the Mississippian, which is at only about 4100
feet below the surface, clearly not in the deep-burial environment (at
least, not today). These minerals may have been emplaced along the
pre-Pennsylvanian unconformity by fluids that evolved within and
subsequently migrated out of the Anadarko Basin.
 Porosity Porosity formed in the mesogenetic environment is represented by the same types
of pores that can form in the eogenetic and telogenetic freshwater
environment (Mazzullo and Harris, 1992), including even cavernous
formed in the mesogenetic environment is represented by the same types
of pores that can form in the eogenetic and telogenetic freshwater
environment (Mazzullo and Harris, 1992), including even cavernous
 porosity porosity (e.g., Hall, 1990; Hill, 1992), which otherwise is generally
known as A burial karst. Therefore, an important point to
remember in this regard is that the diagenetic environment in which (e.g., Hall, 1990; Hill, 1992), which otherwise is generally
known as A burial karst. Therefore, an important point to
remember in this regard is that the diagenetic environment in which
 porosity porosity formation occurred cannot be determined on the basis of the
pore types present in a reservoir! How, then, does one recognize formation occurred cannot be determined on the basis of the
pore types present in a reservoir! How, then, does one recognize
 porosity porosity formed in the mesogenetic environment? The answer to this
question is "With careful, detailed petrographic study of
thin-sections, often combined with analysis of carbon and oxygen
isotopic values of formed in the mesogenetic environment? The answer to this
question is "With careful, detailed petrographic study of
thin-sections, often combined with analysis of carbon and oxygen
isotopic values of  carbonate carbonate cements associated with suspected
mesogenetic cements associated with suspected
mesogenetic  porosity porosity .” For details, refer to the papers by Moore and
Druckman (1981), Druckman and Moore (1985), Heydari and Moore (1989),
Moore (1989) and Mazzullo and Harris (1991, 1992), and the many papers
cited therein. There are, however, some readily visible clues to the
mesogenetic origin of .” For details, refer to the papers by Moore and
Druckman (1981), Druckman and Moore (1985), Heydari and Moore (1989),
Moore (1989) and Mazzullo and Harris (1991, 1992), and the many papers
cited therein. There are, however, some readily visible clues to the
mesogenetic origin of  porosity porosity in in  carbonate carbonate rocks that can be identified
in cuttings and core samples. For example: (1) rocks that can be identified
in cuttings and core samples. For example: (1)  porosity porosity along and
associated with stylolites or which cuts across stylolites (Figure 13).
As pressure-dissolution features, stylolites form most commonly in the
deep-burial environment. So, if the timing of along and
associated with stylolites or which cuts across stylolites (Figure 13).
As pressure-dissolution features, stylolites form most commonly in the
deep-burial environment. So, if the timing of  porosity porosity formation can be
determined by the law of cross-cutting relationships to be younger
than the stylolites, and the rocks are still buried, then formation can be
determined by the law of cross-cutting relationships to be younger
than the stylolites, and the rocks are still buried, then  porosity porosity formation must be the result of mesogenetic dissolution; (2) pores that
cut across cements that contain hydrocarbon inclusions; (3) pores that
cut across or which are intimately associated with fluorite, metal
sulfides such as galena and sphalerite, and sometimes pyrite/marcasite,
which are common Mississippian Valley-type precipitates from migrating
deep-basinal fluids (e.g., Cathles and Smith, 1983; Clendenin and Duane,
1990); and (4) pores that cut across saddle dolomite (Figure 14). Saddle
dolomite, otherwise known as baroque dolomite or “pearl spar," is a
cement or replacive mineral that is readily identified by its
characteristic curved crystal faces, common opaque-white color, and
relatively coarse crystal size, that commonly forms in the deep-burial
environment (Radke and Mathis, 1980). Hence, if pores cut across saddle
dolomite, then
formation must be the result of mesogenetic dissolution; (2) pores that
cut across cements that contain hydrocarbon inclusions; (3) pores that
cut across or which are intimately associated with fluorite, metal
sulfides such as galena and sphalerite, and sometimes pyrite/marcasite,
which are common Mississippian Valley-type precipitates from migrating
deep-basinal fluids (e.g., Cathles and Smith, 1983; Clendenin and Duane,
1990); and (4) pores that cut across saddle dolomite (Figure 14). Saddle
dolomite, otherwise known as baroque dolomite or “pearl spar," is a
cement or replacive mineral that is readily identified by its
characteristic curved crystal faces, common opaque-white color, and
relatively coarse crystal size, that commonly forms in the deep-burial
environment (Radke and Mathis, 1980). Hence, if pores cut across saddle
dolomite, then  porosity porosity formation must be mesogenetic if the rocks are
still buried. Are there examples of mesogenetic formation must be mesogenetic if the rocks are
still buried. Are there examples of mesogenetic  porosity porosity in in  carbonate carbonate reservoirs in Kansas? Undoubtedly, but specific published instances
where mesogenetic
reservoirs in Kansas? Undoubtedly, but specific published instances
where mesogenetic  porosity porosity is present are not available. is present are not available.
Microporosity, Pinpoint  Porosity Porosity , and Chalky , and Chalky  Porosity Porosity
The term
microporosity refers to any very small pores that can be
recognized only with the aid of a high-powered binocular microscope or
thin-section (Choquette and Pray, 1970; Pittman, 1971). Micropores,
otherwise known as pinpoint pores, may variously represent: (1) birdseye
pores in tidal flat deposits; (2) intraparticle pores within small
particles; (3) intercrystalline pores between dolomite crystals or
between calcite cement crystals; (4) intercrystalline pores within the
nuclei or cortices of oolites; or (5) intracrystalline pores within
individual dolomite or calcite cement crystals. In some cases, whereas
matrix microporosity/pinpoint  porosity porosity may not be very permeable for
oil, it very well may be permeable enough for natural gas (e.g., Roehl,
1985; Ruzyla and Friedman, 1985). Chalky may not be very permeable for
oil, it very well may be permeable enough for natural gas (e.g., Roehl,
1985; Ruzyla and Friedman, 1985). Chalky  porosity porosity is a term that
refers to microporosity that commonly forms in highly weathered or
otherwise highly diagenetically altered is a term that
refers to microporosity that commonly forms in highly weathered or
otherwise highly diagenetically altered  carbonate carbonate rocks (Pittman, 1970;
Reeckmann and Friedman, 1982) that, as a consequence of being strongly
altered, are very soft. Although true chalks (i.e., those limestones of
Cretaceous to Paleogene age that are composed dominantly of coccoliths)
commonly contain microporosity, the presence of chalky rocks (Pittman, 1970;
Reeckmann and Friedman, 1982) that, as a consequence of being strongly
altered, are very soft. Although true chalks (i.e., those limestones of
Cretaceous to Paleogene age that are composed dominantly of coccoliths)
commonly contain microporosity, the presence of chalky  porosity porosity does not
necessarily indicate that the rocks under consideration are chalks. In
fact, tripolitic cherts commonly have microporosity, and because the
rocks are relatively soft, such does not
necessarily indicate that the rocks under consideration are chalks. In
fact, tripolitic cherts commonly have microporosity, and because the
rocks are relatively soft, such  porosity porosity can be referred to as chalky. I
point out that microporosity, and specifically chalky can be referred to as chalky. I
point out that microporosity, and specifically chalky  porosity porosity , can form
in , can form
in  carbonate carbonate rocks (and cherts) beneath unconformities as well as in
deeply buried rocks, and as such, their presence does not necessarily
imply the existence of a stratigraphically-nearby unconformity. rocks (and cherts) beneath unconformities as well as in
deeply buried rocks, and as such, their presence does not necessarily
imply the existence of a stratigraphically-nearby unconformity.
Secondary
 porosity porosity in in  carbonate carbonate rocks, in both limestone and dolomite, can be
formed by: (1) freshwater dissolution either in the subunconformity
meteoric, eogenetic or telogenetic environment, or; (2) by dissolution
by chemically aggressive subsurface fluids, generated during maturation
of organic matter in source rocks, in the deep-burial (mesogenetic)
environment. Although pore types formed in these environments are
similar, their origin often can be determined by careful observation,
thin-section petrography, and stable carbon-oxygen isotope analysis. rocks, in both limestone and dolomite, can be
formed by: (1) freshwater dissolution either in the subunconformity
meteoric, eogenetic or telogenetic environment, or; (2) by dissolution
by chemically aggressive subsurface fluids, generated during maturation
of organic matter in source rocks, in the deep-burial (mesogenetic)
environment. Although pore types formed in these environments are
similar, their origin often can be determined by careful observation,
thin-section petrography, and stable carbon-oxygen isotope analysis.
Bathurst, R.G.C., 1980, Deep crustal
diagenesis in limestones: Revista Instituto Investaciones Geologicas,
University of Barcelona, v. 34, p. 89-100.
Burruss, R.C., Cercone, K.R., and
Harris, P.M., 1985, Timing of hydrocarbon maturation - evidence from
fluid inclusions in calcite cements, tectonics and burial history, in
N. Schneidermann and P.M. Harris, eds.,  Carbonate Carbonate Cements: SEPM Special
Publ. 36, p. 277-289. Cements: SEPM Special
Publ. 36, p. 277-289.
Cathles, L.M., and Smith, A.T., 1983,
Thermal constraints on the formation of Mississippi Valley-type
lead-zinc deposits and their implications for episodic basin dewatering
and deposit genesis: Economic Geology, v. 78, p. 983-1002.
Chilingarian, G.V., Torabzadeh, J., Rieke, H.H.,
Metghalchi, M., and Mazzullo, S.J., 1992, Interrelationships
among surface area,
 permeability permeability , ,  porosity porosity , pore size, and residual water saturation, in G.V.
Chilingarian, S.J. Mazzullo, and H.H. Rieke, eds., , pore size, and residual water saturation, in G.V.
Chilingarian, S.J. Mazzullo, and H.H. Rieke, eds.,  Carbonate Carbonate Reservoir
Characterization: A Geologic-Engineering Analysis, Part I: Elsevier Publ.
Co., Amsterdam, Developments in Petroleum Science 30, p. 379-397. Reservoir
Characterization: A Geologic-Engineering Analysis, Part I: Elsevier Publ.
Co., Amsterdam, Developments in Petroleum Science 30, p. 379-397.
Choquette, P.W., and James, N.P.,
1987, Diagenesis 12: diagenesis in limestones-3. The deep burial
environment: Geoscience Canada, v. 14, p. 3-35.
Choquette, P.W., and Pray, L.C., 1970,
Geologic nomenclature and classification of  porosity porosity in sedimentary
carbonates: AAPG Bulletin, v. 54, p. 207-250. in sedimentary
carbonates: AAPG Bulletin, v. 54, p. 207-250.
Clendenin, C.W., and Duane, M.J.,
1990, Focused fluid flow and Ozark Mississippi Valley-type deposits:
Geology, v. 18, p. 116-119.
Druckman, Y., and Moore, C.H., 1985,
Late subsurface  porosity porosity in a Jurassic grainstone reservoir, Smackover
Formation, Mt. Vernon field, southern Arkansas, in P.O. Roehl and
P.W. Choquette, eds., in a Jurassic grainstone reservoir, Smackover
Formation, Mt. Vernon field, southern Arkansas, in P.O. Roehl and
P.W. Choquette, eds.,  Carbonate Carbonate Petroleum Reservoirs: Springer-Verlag,
New York, p. 371-383. Petroleum Reservoirs: Springer-Verlag,
New York, p. 371-383.
Feazel, C.T., and Schatzinger, R.A.,
1985, Prevention of  carbonate carbonate cementation in petroleum reservoirs, in
N. Schneidermann and P.M. Harris, eds., cementation in petroleum reservoirs, in
N. Schneidermann and P.M. Harris, eds.,  Carbonate Carbonate Cements: SEPM Special
Publ. 36, p. 97-106. Cements: SEPM Special
Publ. 36, p. 97-106.
Foscolos, A.E., 1984, Diagenesis 7.
Catagenesis of argillaceous sedimentary rocks: Geoscience Canada, v. 11,
p. 67-75.
Friedman, G.M., 1964, Early diagenesis
and lithification in  carbonate carbonate sediments: Journal of Sedimentary
Petrology, v. 34, p. 777-813. sediments: Journal of Sedimentary
Petrology, v. 34, p. 777-813.
Gatewood, L.E., 1970, Oklahoma City
field - anatomy of a giant, in M.T. Halbouty, ed., Geology of
Giant Petroleum Fields: AAPG Memoir 14, p. 223-254.
Hall, J.F., 1990, The development of
paleokarst and other solution features in the Mississippian Leadville
Dolomite, central Colorado, in D.W. Beaty, G.P. Landis, and T.B.
Thompson, eds.,  Carbonate Carbonate -Hosted Sulfide Deposits of the Central
Colorado Mineral Belt: Economic Geology Monograph 7, New Haven, Conn.,
Economic Geology Publ., p. 108-117. -Hosted Sulfide Deposits of the Central
Colorado Mineral Belt: Economic Geology Monograph 7, New Haven, Conn.,
Economic Geology Publ., p. 108-117.
Halley, R.B., and Schmoker, J.W.,
1983, High  porosity porosity Cenozoic Cenozoic  carbonate carbonate rocks of south Florida:
progressive loss of rocks of south Florida:
progressive loss of  porosity porosity with depth: AAPG Bulletin, v. 67, p.
191-200. with depth: AAPG Bulletin, v. 67, p.
191-200.
Hanor, .S., 1987, Origin and migration
of subsurface sedimentary brines: SEPM Lecture Notes, Short Course No.
21, 247 p.
Hendrickson, A.R., Thomas, R.L., and
Economides, M.J., 1992, Stimulation of  carbonate carbonate reservoirs, in
G.V. Chilingarian, S.J. Mazzullo, and H.H. Rieke, eds., reservoirs, in
G.V. Chilingarian, S.J. Mazzullo, and H.H. Rieke, eds.,  Carbonate Carbonate Reservoir Characterization: A Geologic-Engineering Analysis, Part I:
Elsevier Publ. Co., Amsterdam, Developments in Petroleum Science 30, p.
589-625 .
Reservoir Characterization: A Geologic-Engineering Analysis, Part I:
Elsevier Publ. Co., Amsterdam, Developments in Petroleum Science 30, p.
589-625 .
Heydari, E., and Moore, C.H., 1989,
Burial diagenesis and thermochemical sulfate reduction, Smackover
Formation, southeastern Mississippi salt basin: Geology, v. 17, p.
1080-1084.
Hill, C.A., 1992, Sulfuric acid
oil-field karst, in M.P. Candelaria and C.L. Reed, eds.,
Paleokarst, Karst Related Diagenesis and Reservoir Development: Examples
from Ordovician-Devonian age strata of West Texas and the Mid-Continent:
Permian Basin Section SEPM Publ. 92-33, p. 192-194.
Holtz, M.H., and Kerans, C., 1992,
Characterization and categorization of west Texas Ellenburger
reservoirs, in M.P. Candelaria and C.L. Reed, eds., Paleokarst,
Karst Related Diagenesis and Reservoir Development: Examples from
Ordovician-Devonian age strata of West Texas and the Mid-Continent:
Permian Basin Section SEPM Publ. 92-33, p. 45-54.
Honarpour, M.M., Chilingarian, G.V.,
and Mazzullo, S.J., 1992,  Permeability Permeability and relative and relative  permeability permeability of of
 carbonate carbonate reservoirs, in G.V. Chilingarian, S.J. Mazzullo, and
H.H. Rieke, eds., reservoirs, in G.V. Chilingarian, S.J. Mazzullo, and
H.H. Rieke, eds.,  Carbonate Carbonate Reservoir Characterization: A
Geologic-Engineering Analysis, Part I: Elsevier Publ. Co., Amsterdam,
Developments in Petroleum Science 30, p. 399-416. Reservoir Characterization: A
Geologic-Engineering Analysis, Part I: Elsevier Publ. Co., Amsterdam,
Developments in Petroleum Science 30, p. 399-416.
Jodry, R.L., 1992, Pore geometry of
 carbonate carbonate rocks and capillary pressure curves (basic geologic concepts),
in G.V. Chilingarian, S.J. Mazzullo, and H.H. Rieke, eds., rocks and capillary pressure curves (basic geologic concepts),
in G.V. Chilingarian, S.J. Mazzullo, and H.H. Rieke, eds.,
 Carbonate Carbonate Reservoir Characterization: A Geologic-Engineering Analysis,
Part I: Elsevier Publ. Co., Amsterdam, Developments in Petroleum Science
30, p. 331-377. Reservoir Characterization: A Geologic-Engineering Analysis,
Part I: Elsevier Publ. Co., Amsterdam, Developments in Petroleum Science
30, p. 331-377.
Kharaka, Y.K., Law, L.M., Carothers,
W.C., and Goerlitz, D.F., 1986, Role of organic species dissolved in
formation waters from sedimentary basins in mineral diagenesis, in
D.L. Gautier, ed., Roles of Organic Matter in Sediment Diagenesis: SEPM
Special Publ. 38, p. 111-122.
Land, L.S., 1967, Diagenesis of
skeletal carbonates: Journal of Sedimentary Petrology, v. 37, p.
914-930.
Longman, M.W., 1980,  Carbonate Carbonate diagenetic textures from near surface diagenetic environments: AAPG
Bulletin, v. 64, p. 461-487.
diagenetic textures from near surface diagenetic environments: AAPG
Bulletin, v. 64, p. 461-487.
Lundegard, P.D., and Land, L.S., 1986,
Carbon dioxide and inorganic acids: their role in  porosity porosity enhancement
and cementation, Paloegene of the of the Texas Gulf Coast, in D.L.
Gautier ed., Roles of Organic Matter in Sediment Diagenesis: SEPM
Special Publ. 38, p. 129-146. enhancement
and cementation, Paloegene of the of the Texas Gulf Coast, in D.L.
Gautier ed., Roles of Organic Matter in Sediment Diagenesis: SEPM
Special Publ. 38, p. 129-146.
Mazzullo, S.J., 1994, Models of
 porosity porosity evolution in Permian periplatform evolution in Permian periplatform  carbonate carbonate reservoirs
(debris-flows and turbidites) in the Permian Basin: West Texas
Geological Society Bulletin, v. 34, no. 1, p. 5-12. reservoirs
(debris-flows and turbidites) in the Permian Basin: West Texas
Geological Society Bulletin, v. 34, no. 1, p. 5-12.
Mazzullo, S.J., and Chilingarian, G.V.,
1992, Diagenesis and origin of  porosity porosity , in G.V. Chilingarian,
S.J. Mazzullo, and H.H. Rieke, eds., , in G.V. Chilingarian,
S.J. Mazzullo, and H.H. Rieke, eds.,  Carbonate Carbonate Reservoir
Characterization: A Geologic-Engineering Analysis, Part I: Elsevier Publ.
Co., Amsterdam, Developments in Petroleum Science 30, p. 199-270. Reservoir
Characterization: A Geologic-Engineering Analysis, Part I: Elsevier Publ.
Co., Amsterdam, Developments in Petroleum Science 30, p. 199-270.
Mazzullo, S.J., and Chilingarian, G.V.,
1996, Hydrocarbon reservoirs in karsted  carbonate carbonate rocks, in G.V.
Chilingarian, S.J. Mazzullo, and H.H.Rieke, eds., rocks, in G.V.
Chilingarian, S.J. Mazzullo, and H.H.Rieke, eds.,  Carbonate Carbonate Reservoir
Characterization: A Geologic-Engineering Analysis, Part II: Elsevier
Publ. Co., Amsterdam, Developments in Petroleum Science 44, p. 797-865 . Reservoir
Characterization: A Geologic-Engineering Analysis, Part II: Elsevier
Publ. Co., Amsterdam, Developments in Petroleum Science 44, p. 797-865 .
Mazzullo, S.J., and Harris, P.M.,
1991, An overview of dissolution  porosity porosity development in the deep-burial
environment, with examples from development in the deep-burial
environment, with examples from  carbonate carbonate reservoirs in the Permian
Basin, in M. Candelaria, ed., Permian Basin Plays – Tomorrow’s
Technology Today: West Texas Geological Society, Publ. No. 91-89, p.
125-138. reservoirs in the Permian
Basin, in M. Candelaria, ed., Permian Basin Plays – Tomorrow’s
Technology Today: West Texas Geological Society, Publ. No. 91-89, p.
125-138.
Mazzullo, S.J., and Harris, P.M.,
1992, Mesogenetic dissolution: its role in  porosity porosity development in development in
 carbonate carbonate reservoirs: AAPG Bulletin, v. 76, p. 607-620. reservoirs: AAPG Bulletin, v. 76, p. 607-620.
Meshri, I.D., 1986, On the reactivity
of carbonic and organic acids and generation of secondary  porosity porosity ,
in D.
L. Gautier, ed., Roles of Organic Matter in Sediment
Diagenesis: SEPM Special Publ. 38, p. 123-128. ,
in D.
L. Gautier, ed., Roles of Organic Matter in Sediment
Diagenesis: SEPM Special Publ. 38, p. 123-128.
Moore, C.H., 1989,  Carbonate Carbonate Diagenesis and
Diagenesis and  Porosity Porosity : Elsevier Publ. Co., Developments in
Sedimentology 46, 338 p. : Elsevier Publ. Co., Developments in
Sedimentology 46, 338 p.
Moore, C.H., and Druckman, Y., 1981,
Burial diagenesis and  porosity porosity evolution, Upper Jurassic Smackover,
Arkansas and Louisiana: AAPG Bulletin, v. 65, p. 597-628. evolution, Upper Jurassic Smackover,
Arkansas and Louisiana: AAPG Bulletin, v. 65, p. 597-628.
Murray, R.C., 1960, Origin of  porosity porosity in
in  carbonate carbonate rocks: Journal of Sedimentary Petrology, v. 30, p. 59-84. rocks: Journal of Sedimentary Petrology, v. 30, p. 59-84.
Newell, K.D., Watney, W.L., Cheng,
S.W.L., and Brownrigg, R.L., 1987, Stratigraphic and spatial
distribution of oil and gas production in Kansas: Kansas Geological
Survey, Subsurface Geology Series 9, 86 p.
Pittman, E.D., 1971, Microporosity in
 carbonate carbonate rocks: AAPG Bulletin, v. 55, p. 1873-1878. rocks: AAPG Bulletin, v. 55, p. 1873-1878.
Radke, B.M., and Mathis, R.C., 1980,
On the formation and occurrence of saddle dolomite: Journal of
Sedimentary Petrology, v. 50, p. 1149-1168.
Reeckmann, A., and Friedman, G.M.,
1982, Exploration for  Carbonate Carbonate Petroleum Reservoirs: John Wiley and
Sons, New York, 213 p. Petroleum Reservoirs: John Wiley and
Sons, New York, 213 p.
Roehl, P.O., 1985, Depositional and
diagenetic controls on reservoir rock development and petrophysics in
Silurian tidalites, Interlake Formation, Cabin Creek field area,
Montana, in P.O. Roehl and P.W. Choquette, eds.,  Carbonate Carbonate Petroleum Reservoirs: Springer-Verlag, Berlin, p. 85-105.
Petroleum Reservoirs: Springer-Verlag, Berlin, p. 85-105.
Ruzyla, K., and Friedman, G.M., 1985,
Factors controlling  porosity porosity in dolomite reservoirs of the Ordovician
Red River Formation, Cabin Creek Field, Montana, in P.O. Roehl
and P.W. Choquette, eds., in dolomite reservoirs of the Ordovician
Red River Formation, Cabin Creek Field, Montana, in P.O. Roehl
and P.W. Choquette, eds.,  Carbonate Carbonate Petroleum Reservoirs: Springer-Verlag,
Berlin, p. 41-58. Petroleum Reservoirs: Springer-Verlag,
Berlin, p. 41-58.
Sassen, R., and Moore, C.H., 1988,
Framework of hydrocarbon generation and destruction in eastern Smackover
trend: AAPG Bulletin, v. 72, p. 649-663.
Scholle, P.A., and Halley, R.B., 1985,
Burial diagenesis: Out of sight, out of mind!, in N.
Schneidermann and P.
M. Harris, eds.,  Carbonate Carbonate Cements:
SEPM Special Publ. 36, p. 309-334. Cements:
SEPM Special Publ. 36, p. 309-334.
Surdam, R.C., Boese, S.W., and Crossey,
L.J., 1984, The chemistry of secondary  porosity porosity , in D.A. McDonald
and R.C. Surdam, eds., Clastic Diagenesis: AAPG Memoir 37, p. 127-149. , in D.A. McDonald
and R.C. Surdam, eds., Clastic Diagenesis: AAPG Memoir 37, p. 127-149.
Walters, R.F., 1946, Buried
pre-Cambrian hills in northeastern Barton County, central Kansas: AAPG
Bulletin, v. 30, p. 660-710.
Wardlaw, N.C., 1996, Factors affecting
oil recovery from  carbonate carbonate reservoirs and prediction of recovery, in
G.
V. Chilingarian, S.J. Mazzullo, and H.H. Rieke, eds., reservoirs and prediction of recovery, in
G.
V. Chilingarian, S.J. Mazzullo, and H.H. Rieke, eds.,
 Carbonate Carbonate Reservoir Characterization: A Geologic-Engineering Analysis,
Part II: Elsevier Publ. Co., Amsterdam, Developments in Petroleum
Science 44, p. 867-903 . Reservoir Characterization: A Geologic-Engineering Analysis,
Part II: Elsevier Publ. Co., Amsterdam, Developments in Petroleum
Science 44, p. 867-903 .
Wardlaw, N.C., 1976, Pore geometry of
 carbonate carbonate rocks as revealed by pore casts and capillary pressures: AAPG
Bulletin, v. 60, p. 245-257. rocks as revealed by pore casts and capillary pressures: AAPG
Bulletin, v. 60, p. 245-257.
Weyl, P.K., 1960,  Porosity Porosity through
dolomitization: conservation-of-mass requirements: Journal of
Sedimentary Petrology, v. 30, p. 85-90. through
dolomitization: conservation-of-mass requirements: Journal of
Sedimentary Petrology, v. 30, p. 85-90.
Wilhite, B.W., and Mazzullo, S.J.,
2000, Facies architecture and diagenesis of Holocene  carbonate carbonate sands in
an inner-platform environment: analog of some ancient sands in
an inner-platform environment: analog of some ancient  carbonate carbonate reservoirs, in S.T. Reid, ed., Transactions of Southwest Section
AAPG, Publ. SWS 2000-107, p. 67-79.
reservoirs, in S.T. Reid, ed., Transactions of Southwest Section
AAPG, Publ. SWS 2000-107, p. 67-79.
Wilhite, B.W., and Mazzullo, S.J.,
2000, Facies architecture and diagenesis of Holocene  carbonate carbonate sands in
an inner-platform environment: analog of some ancient sands in
an inner-platform environment: analog of some ancient  carbonate carbonate reservoirs, in S.T. Reid, ed., Transactions of Southwest Section
AAPG, Publ. SWS 2000-107, p. 67-79.
reservoirs, in S.T. Reid, ed., Transactions of Southwest Section
AAPG, Publ. SWS 2000-107, p. 67-79.
Return to top.
|
 Click to view article as PDF
Click to view article as PDF Porosity
Porosity Evolution in
Evolution in  Carbonate
Carbonate Reservoirs*
Reservoirs* Carbonate
Carbonate rocks (limestone and
dolomite) account for approximately 50% of oil and gas production around the
world. Of the carbonates, a slightly greater percentage of world hydrocarbon
reserves has been produced from dolomites because such rocks commonly, but not
always, have more
rocks (limestone and
dolomite) account for approximately 50% of oil and gas production around the
world. Of the carbonates, a slightly greater percentage of world hydrocarbon
reserves has been produced from dolomites because such rocks commonly, but not
always, have more  porosity
porosity and
and  permeability
permeability than limestones (Halley and Schmoker,
1983). Unlike most sandstone reservoirs, which typically are single-
than limestones (Halley and Schmoker,
1983). Unlike most sandstone reservoirs, which typically are single- porosity
porosity systems (i.e., interparticle pores) of relative uniform (homogeneous) nature,
reservoirs in
systems (i.e., interparticle pores) of relative uniform (homogeneous) nature,
reservoirs in  carbonate
carbonate rocks commonly are multiple-
rocks commonly are multiple- porosity
porosity systems that
characteristically impart petrophysical heterogeneity to the reservoirs (Mazzullo
and Chilingarian, 1992). Hence, the specific types and relative percentages of
pores present, and their distribution within the rocks, exert strong control on
the production and stimulation characteristics of
systems that
characteristically impart petrophysical heterogeneity to the reservoirs (Mazzullo
and Chilingarian, 1992). Hence, the specific types and relative percentages of
pores present, and their distribution within the rocks, exert strong control on
the production and stimulation characteristics of  carbonate
carbonate reservoirs (e.g.,
Jodry, 1992; Chilingarian et al., 1992; Honarpour et al., 1992; Hendrickson et
al., 1992; Wardlaw, 1996). Pore types in
reservoirs (e.g.,
Jodry, 1992; Chilingarian et al., 1992; Honarpour et al., 1992; Hendrickson et
al., 1992; Wardlaw, 1996). Pore types in  carbonate
carbonate rocks can generally be
classified on the basis of the timing of
rocks can generally be
classified on the basis of the timing of  porosity
porosity evolution (Choquette
and Pray, 1970) into: (1) primary pores (or depositional
evolution (Choquette
and Pray, 1970) into: (1) primary pores (or depositional  porosity
porosity ), which are
pores inherent in newly-deposited sediments and the particles that
comprise them. Such pore types include interparticle pores in, for
example,
), which are
pores inherent in newly-deposited sediments and the particles that
comprise them. Such pore types include interparticle pores in, for
example,  carbonate
carbonate sands (but also in muddy carbonates), intraparticle
pores (within particles such as foraminifera or gastropod shells), fenestral
pores (formed by gas bubbles and sediment shrinkage in tidal-flat carbonates),
and shelter and growth-framework pores (common in reef buildups);
and (2) secondary pores, which are those that form as a result of later,
generally post-depositional dissolution. Such pore types include all of those
mentioned above, but only when it can be demonstrated that primary pores which
subsequently were occluded by cement later had all or some of that cement
dissolved (resulting in the generation of exhumed pores - Figure 2), as well as
vugs (large pores that transect rock fabric, that is, dissolution was not
fabric-selective) and dissolution-enlarged fractures. Most of these
primary and secondary pore types can readily be identified in cores, and with
the possible exception of shelter and growth-framework pores, also in well
cuttings samples.
sands (but also in muddy carbonates), intraparticle
pores (within particles such as foraminifera or gastropod shells), fenestral
pores (formed by gas bubbles and sediment shrinkage in tidal-flat carbonates),
and shelter and growth-framework pores (common in reef buildups);
and (2) secondary pores, which are those that form as a result of later,
generally post-depositional dissolution. Such pore types include all of those
mentioned above, but only when it can be demonstrated that primary pores which
subsequently were occluded by cement later had all or some of that cement
dissolved (resulting in the generation of exhumed pores - Figure 2), as well as
vugs (large pores that transect rock fabric, that is, dissolution was not
fabric-selective) and dissolution-enlarged fractures. Most of these
primary and secondary pore types can readily be identified in cores, and with
the possible exception of shelter and growth-framework pores, also in well
cuttings samples.
 carbonate
carbonate sediments is that primary
sediments is that primary  porosity
porosity is substantially reduced by
cementation and compaction during post-depositional burial (Figure 1; Halley and
Schmoker, 1983), many workers would argue that most
is substantially reduced by
cementation and compaction during post-depositional burial (Figure 1; Halley and
Schmoker, 1983), many workers would argue that most  porosity
porosity in limestone and
dolomite reservoirs is of secondary origin (e.g., Mazzullo and Chilingarian,
1992). Exceptions to this statement are cases where primary
in limestone and
dolomite reservoirs is of secondary origin (e.g., Mazzullo and Chilingarian,
1992). Exceptions to this statement are cases where primary  porosity
porosity is
preserved because of the early influx of hydrocarbons into pores (e.g., Feazel
and Schatzinger, 1985). Early on in the study of
is
preserved because of the early influx of hydrocarbons into pores (e.g., Feazel
and Schatzinger, 1985). Early on in the study of  carbonate
carbonate sediments and their
diagenesis, the subaerial meteoric diagenetic (freshwater) model was
promoted as a means of explaining
sediments and their
diagenesis, the subaerial meteoric diagenetic (freshwater) model was
promoted as a means of explaining  porosity
porosity evolution in carbonates, specifically
in shallow-water carbonates that lie beneath unconformities in paleo-vadose and
paleo-phreatic freshwater zones (e.g., Friedman, 1964; Land, 1967). This model
still is heavily applied today, especially in sequence stratigraphic-related
diagenetic studies of reservoirs. This model presupposes that if porous
evolution in carbonates, specifically
in shallow-water carbonates that lie beneath unconformities in paleo-vadose and
paleo-phreatic freshwater zones (e.g., Friedman, 1964; Land, 1967). This model
still is heavily applied today, especially in sequence stratigraphic-related
diagenetic studies of reservoirs. This model presupposes that if porous
 carbonate
carbonate rocks are present beneath unconformities, then that
rocks are present beneath unconformities, then that  porosity
porosity must have
been created by freshwater dissolution during subaerial exposure. Of course, as
explorationists, we all can probably list a large number of wells that were
drilled into non-porous
must have
been created by freshwater dissolution during subaerial exposure. Of course, as
explorationists, we all can probably list a large number of wells that were
drilled into non-porous  carbonate
carbonate rocks beneath unconformities. Hence, the
corollary pertaining to subaerial exposure is not true - meteoric exposure does
not always create
rocks beneath unconformities. Hence, the
corollary pertaining to subaerial exposure is not true - meteoric exposure does
not always create  porosity
porosity , and even if it did, that
, and even if it did, that  porosity
porosity may be occluded
during later burial (Figure 1). A most critical constraint on evaluating, or
more importantly, on
may be occluded
during later burial (Figure 1). A most critical constraint on evaluating, or
more importantly, on  predicting
predicting
 porosity
porosity in
in  carbonate
carbonate rocks utilizing only the subaerial meteoric diagenetic model is that one must call upon fluids capable of
dissolving
rocks utilizing only the subaerial meteoric diagenetic model is that one must call upon fluids capable of
dissolving  carbonate
carbonate to come from above; that is, from rain water
percolating down into sediments or rocks beneath unconformity surfaces.
Ostensibly, then, many might consider that if there is not an unconformity in
the section, then the carbonates will not be porous. Again, as explorationists
we can all probably compile a list of wells in which porous carbonates that were
not associated with unconformities were encountered in the subsurface.
to come from above; that is, from rain water
percolating down into sediments or rocks beneath unconformity surfaces.
Ostensibly, then, many might consider that if there is not an unconformity in
the section, then the carbonates will not be porous. Again, as explorationists
we can all probably compile a list of wells in which porous carbonates that were
not associated with unconformities were encountered in the subsurface.  porosity
porosity in
in  carbonate
carbonate rocks be
generated by processes other than subaerial meteoric exposure, and if so,
what are those processes?; (2) how might reservoir
rocks be
generated by processes other than subaerial meteoric exposure, and if so,
what are those processes?; (2) how might reservoir  porosity
porosity formed by such
alternative processes differ from reservoirs created by meteoric dissolution
along unconformities?; (3) how can we recognize and determine the origin of
reservoir
formed by such
alternative processes differ from reservoirs created by meteoric dissolution
along unconformities?; (3) how can we recognize and determine the origin of
reservoir  porosity
porosity ?; and (4) can the subsurface occurrence of
?; and (4) can the subsurface occurrence of  porosity
porosity formed by
any such alternative models of reservoir origin be predicted? The purpose of
this contribution is to address these questions by demonstrating the
multi-faceted evolution of secondary
formed by
any such alternative models of reservoir origin be predicted? The purpose of
this contribution is to address these questions by demonstrating the
multi-faceted evolution of secondary  porosity
porosity in
in  carbonate
carbonate hydrocarbon
reservoirs. In the following discussions attention will be focused on the
recognition and origin of pore types in shallow-water limestones and dolomites,
as observed mainly in cores and well cuttings samples.
hydrocarbon
reservoirs. In the following discussions attention will be focused on the
recognition and origin of pore types in shallow-water limestones and dolomites,
as observed mainly in cores and well cuttings samples. 












